
From October 1, 2020, a batch of new medical devices must be assigned with UDI code, having their own "IDs".
In this batch, there are 20 enterprises involved in this kind of products in Hangzhou, accounting for about 1/4 of the total number of medical device enterprises in Hangzhou. The products involved include 10 kinds of products, such as heart valves, blood dialyzer, intraocular lens, balloon catheter, and so on.
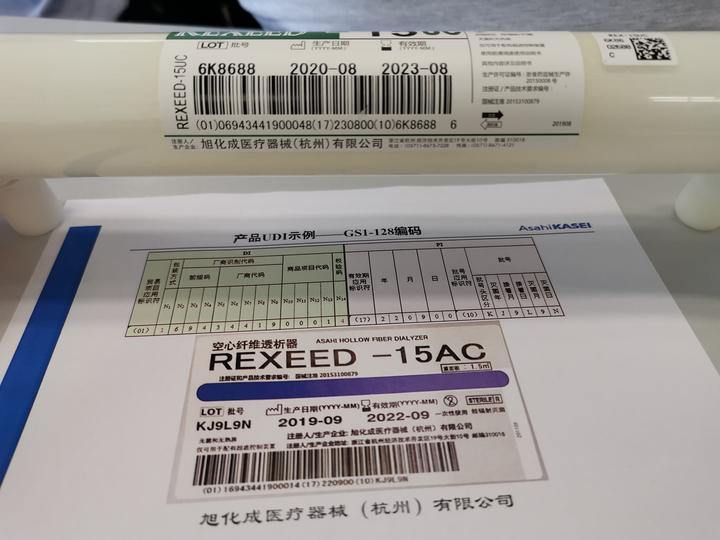
UDI, Unique Device Identification, is the current international uniform identification of medical equipment quality traceability. UDI code is a bar code, composed of numbers, letters or symbols, attached to the product or package of medical devices for the unique identification of medical devices.
"It is the basis for unique and accurate identification of medical devices, which runs through the production, circulation, and use of medical devices, and contributes to the full life cycle management of medical devices." Zhou Jie, director of the medical device supervision department of Hangzhou Market Supervision Bureau, said UDI code includes product identification and product identification.
Product identification is the only code to identify the registrant, medical device type specifications, and packaging. The product label is composed of the codes of the information related to the production process of medical devices. According to the requirements of supervision and practical application, it can contain the serial number, production batch number, production date and expiration date of medical devices, etc.

For the hospital, "with UDI and relying on its information-based management system, it is more convenient for us to manage high-risk consumables". Ying Yue, an engineer of the Clinical Medical Engineering Department of the Second Affiliated Hospital of Zhejiang University School of Medicine, emphasized that, in particular, the imprinted medical consumables require more detailed traceability management because they are kept in the body for a long time. "It used to be manual recording. But in case of adverse reactions, it would be difficult to trace the data and information."
"Medical device manufacturers, distributors, operators, and users can scan the code with professional tools to fully understand the full life cycle information of the product." Zhou Jie introduced, the public can go to the medical device's unique identification management system of National Medical Products Administration for inquiries. "This will help improve the efficiency of regulation, combine government regulation with social governance, and further safeguard the safety of using medical devices."
And to the enterprise, UDI is conducive to forcing the production management to be stricter. At the same time, if there is a problem after the product is sold, the UDI code can be used to quickly trace the information of each production link of the product, which is helpful to the investigation of the cause of the product failure. In addition, UDI code can be used to trace the inventory of consumables of users such as hospitals, so as to achieve accurate sales and use of products.
(Compiled by Xu Yuhong, translated by Yu Fei)